Lecithin is a mixture of phospholipids, glycolipids, glycerides, and other minor components. It is due to its high phospholipid content that lecithin possesses specific functions. Phospholipids are amphiphilic, meaning they have different affinities for oil and water. Lecithin forms stable O/W and W/O emulsions and durable dispersions in a wide range of applications. It is generally found in all living cells as a major component of the cell membrane, which regulates the nutrients entering and exiting the cell or the metabolic processes. Lecithin is a vital feature of all life; it is an essential nutritional supplement.
Lecithin
Lecithin – General Information

What is Lecithin?
Lecithin Production
Vegetable-origin lecithin is derived during the stages of oil refining of soybean, sunflower, rapeseed, or rice bran oil.
Selection of Emulsifiers
How Are Emulsifiers Selected?
One method is by using HLB (Hydrophilic Lipophilic Balance).
Different emulsifiers have different HLB values, and proper emulsifiers are selected depending on the composition of the proposed emulsion.
What Is HLB Value?
HLB stands for Hydrophilic Lipophilic Balance. A low HLB value indicates greater lipophilicity or a higher affinity towards lipids (oil). Conversely, a high HLB value indicates a greater affinity towards water. Emulsifiers with high HLB values are used in O/W type of emulsions, and vice versa.
Types of Emulsions
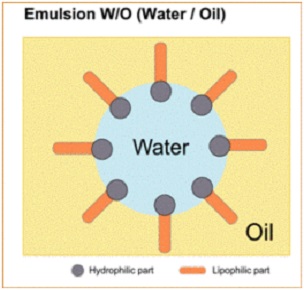
Water in Oil
Water in Oil. Butte, Margarines, oil is in continuous form while water is dispersed in small droplets.
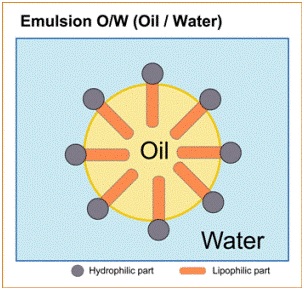
Oil in Water
Oil in Water e.g. Milk, cream (buttercream). Milk and milk fat -water is the continuous phase and fat is in suspended form.
Functions of Emulsifier
Emulsifiers are molecules with groups that exhibit solubility or affinity towards both water and oil. For example, the fatty acid chains in lecithin have an affinity for oil, while the phosphatide chains have an affinity for water due to their polarity. As a result, this molecule can effectively hold together both oil and water phases.